When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. That’s the whole point of bioequivalence - it’s the scientific promise that the generic delivers the same medicine, the same way, to your body. But what if the batch of generic pills you’re holding isn’t even the same as the one tested in the lab? What if the brand-name drug itself changes slightly from batch to batch? This isn’t theoretical. It’s happening right now, and it’s undermining the reliability of bioequivalence testing.
What Bioequivalence Really Means - and What It Doesn’t
Bioequivalence is measured by comparing how your body absorbs the drug. The key numbers are AUC (total exposure) and Cmax (peak concentration). Regulatory agencies like the FDA and EMA say a generic is equivalent if the 90% confidence interval for the ratio of these values between the test (generic) and reference (brand) product falls between 80% and 125%. That’s it. If the numbers land in that range, the product gets approved. No clinical trials needed.
But here’s the problem: this method assumes the reference product is perfectly consistent. It isn’t. Manufacturing isn’t magic. Even the same brand-name drug, made in the same factory, can vary slightly from batch to batch. A 2016 study in Clinical Pharmacology & Therapeutics found that between-batch variability accounts for 40% to 70% of the total error in pharmacokinetic measurements. That’s not noise. That’s a major source of variation - and it’s being ignored.
Why Single-Batch Testing Is a Flawed System
Right now, bioequivalence studies compare one batch of the generic to one batch of the brand. That’s it. But if the brand’s batch happens to be unusually high in potency, and the generic batch is average, the generic might look underperforming - even if it’s perfectly fine. Conversely, if the brand batch is weak and the generic is strong, they might both fall within the 80-125% range, making them appear equivalent when they’re not.
This isn’t just a statistical glitch. It’s a real risk. Researchers call it “confounded bioequivalence.” The outcome depends less on the product’s true performance and more on which two batches you happened to pick for the test. A 2019 presentation by Dr. Robert Lionberger, former head of the FDA’s Office of Generic Drugs, called this “one of the most significant statistical oversights in modern bioequivalence assessment.” He warned it leads to both false positives (approving inequivalent drugs) and false negatives (rejecting good ones).
How Batch Variability Skews the Numbers
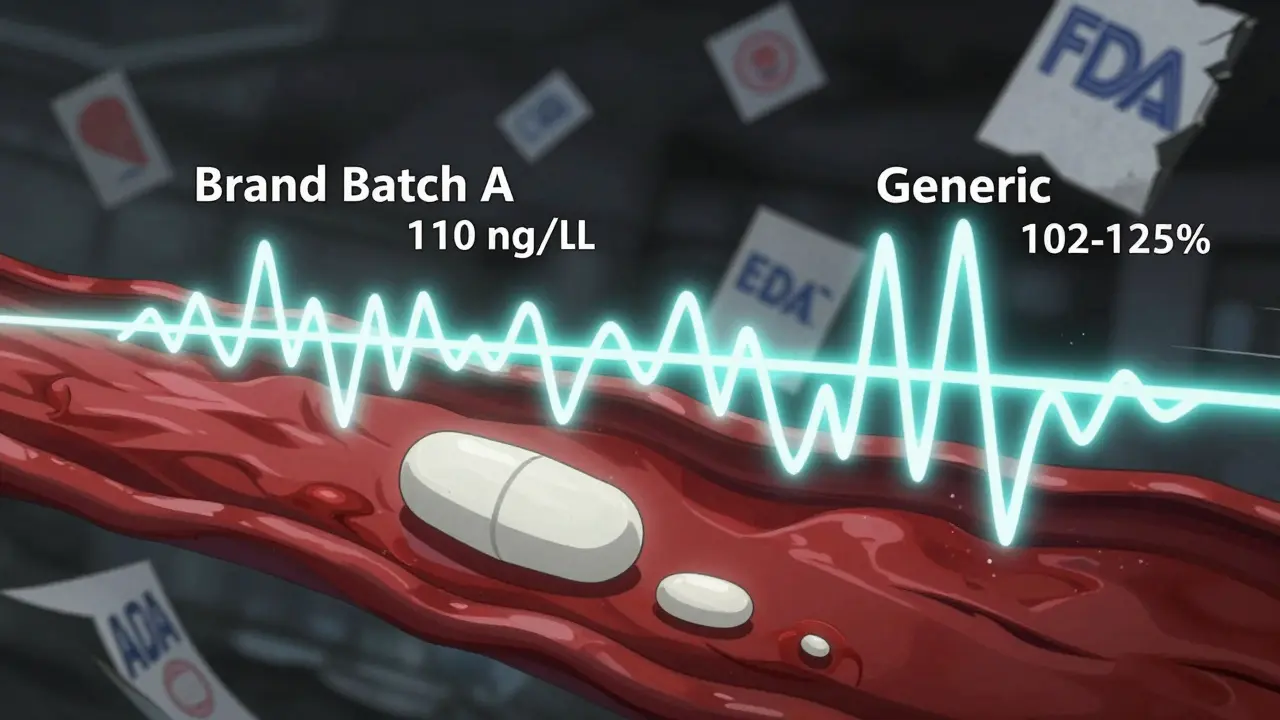
Imagine you’re testing two versions of a nasal spray. The brand’s average Cmax is 100 ng/mL, but batch A reads 110, batch B reads 92, and batch C reads 105. That’s a 18-point swing - nearly 20% variation. Now, the generic is made from a batch that averages 102. If you test it against batch A (110), the ratio is 92.7% - within limits. But test it against batch B (92), and the ratio jumps to 110.9% - still within limits. So, it passes.
But what if you tested the same generic against a different brand batch? The results could swing wildly. The 80-125% rule doesn’t care. It just sees “within range” and approves. Meanwhile, patients might get inconsistent dosing. For drugs with narrow therapeutic windows - like blood thinners or seizure meds - even small shifts matter.

The New Approach: Between-Batch Bioequivalence (BBE)
A better way exists. It’s called Between-Batch Bioequivalence (BBE). Instead of comparing the generic to one brand batch, you compare it to the average variability of multiple brand batches. The test asks: Is the difference between the generic and the average brand batch smaller than the natural variation seen across the brand’s own batches?
The rule is simple: if the absolute difference between the generic and the brand’s average is less than twice the brand’s between-batch standard deviation, they’re considered equivalent. This method doesn’t require more patients. It just requires testing more batches.
Simulations show that with just three reference batches, BBE correctly identifies true equivalence 65% of the time. With six batches, that jumps to over 85%. That’s a huge improvement. The FDA already uses a version of this for budesonide nasal spray. It’s time to make it standard.
What’s Changing in Regulation - and When
Regulators are starting to wake up. In June 2023, the FDA released a draft guidance titled Consideration of Batch-to-Batch Variability in Bioequivalence Studies. It’s not law yet, but it’s a clear signal. The EMA’s 2023 workshop on complex generics listed “inadequate consideration of batch variability” as one of the top three challenges in generic drug approval.
By 2025, experts predict the new norm will be: at least three reference batches and two test batches for complex products like inhalers, nasal sprays, and injectables. The EMA’s 2022 reflection paper already recommends this. The FDA’s 2022 guidance on nasal sprays requires evidence of batch consistency across at least three production-scale batches. And the International Council for Harmonisation (ICH) is working on new guidelines (Q13) that push for better statistical methods across manufacturing scales.
Industry is already catching up. A 2022 survey found 78% of major generic manufacturers now test multiple batches for complex products - up from just 32% in 2018. That’s not because they’re being forced. It’s because they know single-batch testing is unreliable.
What This Means for You - Patient and Prescriber
As a patient, you should know: not all generics are created equal - and not all approvals are created equal. The current system gives you confidence, but that confidence is built on shaky ground. If you’ve ever noticed a change in how a generic drug works after a refill, you’re not imagining it. Batch variability might be why.
As a prescriber, you can’t always control which generic your patient gets. But you can ask: Is this a simple tablet, or a complex product like an inhaler or extended-release capsule? For the latter, batch variability matters more. If a patient reports unexpected side effects or reduced effectiveness after switching generics, consider batch differences as a possible cause - not just non-adherence.
What’s Next? The Future of Generic Drug Approval
The 80-125% rule was a breakthrough in the 1990s. It made generics affordable and accessible. But it was designed for simple, well-behaved drugs. Today’s generics include complex formulations - liposomes, nanoparticles, inhalers, transdermal patches - where small manufacturing changes have big clinical impacts.
Continuing to use single-batch bioequivalence for these products is like judging two cars by test-driving one from each factory and ignoring that each factory has multiple assembly lines with different tolerances. It’s not just outdated. It’s dangerous.
The shift to multi-batch testing isn’t about making generics harder to approve. It’s about making sure the ones that get approved are truly equivalent - not just statistically lucky. It’s about protecting patients from inconsistent dosing, reducing adverse events, and ensuring that the promise of generic drugs - safe, effective, affordable - stays real.
By 2025, we’ll likely look back at the single-batch era the way we look at using mercury thermometers - a relic of a time when we didn’t know better. The science is here. The regulators are moving. The industry is ready. Now, it’s just a matter of time before the rules catch up.