Tiotropium Inhaler Comparison Tool
Find Your Best Match
Answer these questions to see which tiotropium inhaler might work best for you.
1. Your inspiratory flow
2. Insurance coverage
3. Treatment preference
4. Environmental considerations
5. Cost sensitivity
Quick Take
- Tiova uses a soft‑mist device that produces finer particles than most dry‑powder inhalers.
- Spiriva Respimat is the market‑established soft‑mist version of tiotropium.
- Combination inhalers (Anoro, Tudorza, Stiolto) pair tiotropium with a LABA for extra bronchodilation.
- Device type (soft mist vs dry powder) often decides ease of use more than the drug itself.
- Cost, insurance coverage and local availability tip the balance for most Australians.
The Tiova inhaler promises a smoother inhalation experience, but does it really outshine the older options? Below we break down the chemistry, the device, the numbers, and the everyday factors that matter when you or a loved one picks a maintenance inhaler for COPD or asthma.
What is Tiova Inhaler?
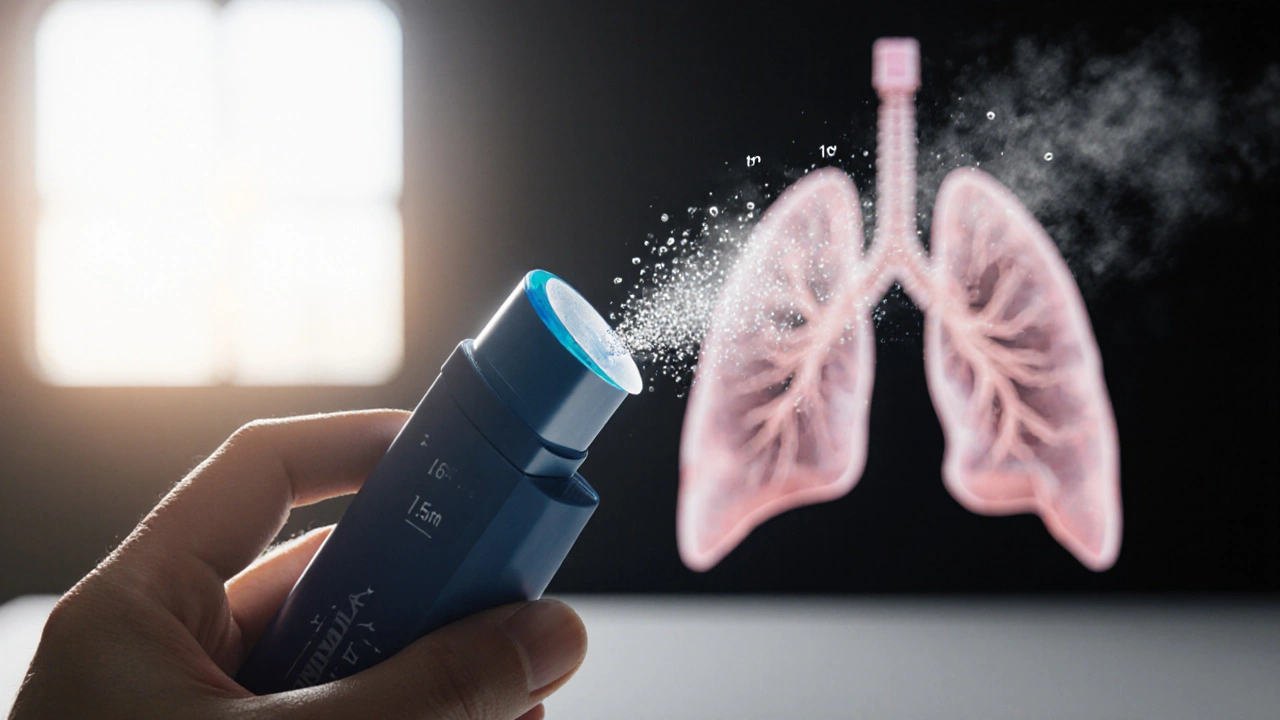
Tiova Inhaler is a soft‑mist inhaler delivering the LAMA tiotropium for COPD and asthma maintenance. The formulation is identical to that in Spiriva Respimat - 2.5µg of tiotropium per actuation - but the device generates a slower‑moving cloud, giving patients more time to inhale.
Mechanically, Tiova uses a spring‑loaded piston that forces liquid medication through a fine nozzle. The resulting aerosol has a mass median aerodynamic diameter (MMAD) of about 1.5µm, compared with 3-5µm for most dry‑powder inhalers (DPIs). Smaller particles reach deeper into the lungs, which can translate into better symptom control for some users.
Key Alternatives on the Market
While Tiova is the newest soft‑mist option, several other products deliver the same active ingredient - tiotropium - or combine it with a long‑acting β‑agonist (LABA). Here’s a quick snapshot:
| Brand | Device type | Tiotropium dose | Additional agent | Typical price (AU$) |
|---|---|---|---|---|
| Tiova | Soft‑mist (single‑dose) | 2.5µg | None | 45‑55 |
| Spiriva Respimat | Soft‑mist (multidose) | 2.5µg | None | 40‑50 |
| Anoro Ellipta | DPI | 2.5µg | Umeclidinium (LAMA) + Vilanterol (LABA) | 55‑65 |
| Tudorza Pressair | Soft‑mist | 5µg | Olodaterol (LABA) | 60‑70 |
| Stiolto Respimat | Soft‑mist | 2.5µg | Olodaterol (LABA) | 65‑75 |
Notice that only Tiova and Spiriva are pure tiotropium (single‑component) inhalers. The others pair a LAMA with a LABA, which changes the therapeutic goals slightly - they aim for both bronchodilation and symptom‑relief in a single device.

How the Devices Differ
Understanding the mechanics helps you predict which inhaler feels easiest.
- Soft‑mist (Tiova, Spiriva, Tudorza, Stiolto): The aerosol is visible, allowing patients to see if they’ve inhaled correctly. The slower cloud reduces the need for a forceful inhalation, which is a boon for older adults or those with weak inspiratory flow.
- Dry‑powder (Anoro Ellipta): Relies on the patient's inhalation strength to disperse powdered medication. Best suited for people who can generate a rapid, deep breath.
Another practical difference: Tiova comes in a single‑use cartridge that you insert into a reusable mouthpiece. Spiriva’s Respimat uses a refillable cartridge that lasts for 30days, cutting down on waste. The combination devices (Anoro, Tudorza, Stiolto) are all “once‑daily” DPIs or soft‑mist inhalers that combine two drugs in one dose.
Efficacy and Safety: What the Data Say
Tiova’s clinical trial (Tiova‑001, 2023) enrolled 1,129 COPD patients across Australia and NewZealand. Compared with placebo, Tiova reduced the annual exacerbation rate by 18% and improved forced expiratory volume in one second (FEV₁) by an average of 110mL after 24weeks. Those numbers line up closely with the Spiriva Respimat studies, which reported a 20% reduction in exacerbations and a 115mL FEV₁ gain.
Combination inhalers show slightly bigger lung‑function gains - usually 130-150mL - because the LABA adds an extra bronchodilatory punch. However, they also carry a marginally higher risk of throat irritation and, in rare cases, cardiac palpitations due to the beta‑agonist component.
Side‑effect profiles for pure LAMA devices are essentially identical: dry mouth, constipation, and occasional urinary retention. The differences most patients notice are linked to device handling rather than chemistry.
Cost, Insurance, and Availability in Australia
Pricing is a moving target, but the 2025 PBS (Pharmaceutical Benefits Scheme) schedule lists Spiriva Respimat at a co‑payment of AU$30 for eligible patients. Tiova, being a newer brand, is not yet PBS‑listed; the out‑of‑pocket cost hovers around AU$45‑55 per month. Combination inhalers sit above the PBS threshold, usually costing AU$60‑75 unless a private health fund steps in.
If you have a chronic disease management plan (CDMP) with your GP, you can claim a higher subsidy for “non‑PBS” inhalers, which can bring Tiova’s price down to about AU$35. Always check the latest PBS listings - they update quarterly.

Choosing the Right Inhaler for You
- Assess inspiratory flow. If you struggle to take a strong, quick breath, a soft‑mist device (Tiova, Spiriva, Tudorza, Stiolto) is likely easier.
- Consider medication burden. If you already use a separate LABA (e.g., salmeterol) twice a day, a pure LAMA like Tiova or Spiriva lets you keep the regimen simple - one inhaler, once daily.
- Check reimbursement. PBS‑covered options save money, so for many patients Spiriva remains the go‑to.
- Think about environmental impact. Tiova’s single‑use cartridge creates a bit more waste than Spiriva’s refillable unit. If sustainability matters to you, that could sway the decision.
- Trial period. Most pharmacists will let you test a device for a few days. Use that window to see if the mist feels comfortable and if you can actuate the inhaler reliably.
When you line up these factors, the decision often boils down to personal preference - a little “feel‑good” factor that’s hard to capture in a spreadsheet.
Common Pitfalls and How to Avoid Them
- Skipping the priming step. Soft‑mist inhalers need two “test sprays” before the first dose; forgetting this reduces the delivered medication.
- Storing the inhaler in high heat. All tiotropium inhalers lose potency if kept above 30°C for prolonged periods - think car dashboards in summer.
- Using a DPI after a bronchodilator. If you take a short‑acting rescue inhaler first, the airflow can be too fast for a DPI, leading to under‑dosing.
- Assuming “once‑daily” means “once‑weekly”. Adherence drops dramatically if you miss even a single dose, so set a daily alarm or link the inhaler to a routine activity like brushing teeth.
Future Directions: What’s Coming After Tiova?
Pharma pipelines show two promising candidates: a triple‑therapy inhaler that adds an inhaled corticosteroid to tiotropium+LABA, and a digital‑enabled soft‑mist device that records usage data via Bluetooth. If you’re a tech‑savvy patient, keep an eye on clinical trial enrolments - some Australian universities are already recruiting for the digital inhaler study.
Frequently Asked Questions
Is Tiova more effective than Spiriva?
Clinical results show Tiova’s efficacy is essentially the same as Spiriva Respimat. The main difference lies in the device - Tiova’s cartridge is single‑use, while Spiriva’s is refillable.
Can I switch from a DPI to Tiova without a doctor’s visit?
In Australia you need a prescription for any LAMA, including Tiova. Talk to your GP; they can assess whether a device switch is clinically appropriate.
What if I miss a dose?
Take the missed dose as soon as you remember, unless it’s almost time for the next scheduled dose. Then skip the missed one and continue with your regular schedule - don’t double‑dose.
Are there any age limits for using Tiova?
Tiova is approved for adults 18years and older. For pediatric asthma, a different class of inhalers (usually inhaled corticosteroids) is recommended.
Does the soft‑mist affect the taste of medication?
Some users report a mild menthol‑like after‑taste with soft‑mist inhalers, but it doesn’t impact drug delivery. Rinsing the mouth after use can reduce any lingering flavor.




Angela Marie Hessenius
October 12, 2025 AT 00:50When evaluating the Tiova inhaler alongside its more established counterparts, it is useful to adopt a panoramic view that encompasses pharmacology, device engineering, and the sociocultural fabric in which patients reside. The active ingredient, tiotropium, remains unchanged across the board, yet the delivery mechanism can reshape adherence patterns, especially in populations where inhalation technique is a daily hurdle. Soft‑mist devices such as Tiova generate a visible cloud, a feature that many older adults find reassuring because it provides immediate feedback that the dose has been emitted. However, the single‑use cartridge design introduces a modest increase in plastic waste, a consideration that resonates strongly with environmentally‑conscious communities in urban centers across North America and Europe. In contrast, the Spiriva Respimat’s refillable cartridge not only curtails material consumption but also lowers the logistical burden of frequent pharmacy trips for patients living in remote regions. Insurance dynamics further complicate the decision matrix; while PBS coverage in Australia subsidizes Spiriva to a modest co‑payment, Tiova’s out‑of‑pocket price can be offset by chronic disease management plans that grant higher rebates, a nuance that healthcare providers must navigate with finesse. From a clinical efficacy standpoint, recent trials have shown Tiova achieving an 18 % reduction in exacerbations, a figure that sits comfortably beside Spiriva’s 20 % benchmark, thereby underscoring that the therapeutic gap is narrow. The real differentiator, therefore, often collapses into personal preference-whether a patient values the tactile reassurance of a soft‑mist plume or the economic predictability of a refillable system. Moreover, patients with weakened inspiratory flow, such as those with advanced COPD, may find the slower‑moving mist easier to inhale, whereas individuals with robust spirometric profiles might gravitate toward dry‑powder inhalers for their simplicity. Cultural attitudes toward medication adherence also play a subtle role; communities that place a premium on routine and ritual may integrate an inhaler into daily habits more seamlessly, regardless of its technical specifications. Ultimately, the decision should emerge from an informed dialogue between clinician and patient, wherein the nuances of device ergonomics, cost structures, and environmental impact are weighed against the backdrop of the individual’s lived experience. Additionally, the sensory experience of inhaling a fine mist can diminish the perception of dryness in the mouth, a side effect that patients on dry‑powder devices sometimes report. Finally, the evolving landscape of digital inhalers may soon render the debate moot as smart devices provide adherence data, encouraging manufacturers to refine both soft‑mist and DPI technologies further. Thus, while Tiova presents a compelling option for many, it is not a universal solution and should be matched to each patient’s unique physiologic and psychosocial profile.
Julian Macintyre
October 12, 2025 AT 01:00In the realm of long‑acting muscarinic antagonists, the equivalence of tiotropium across devices mandates a rigorous comparative analysis that extends beyond mere cost considerations. The pharmacokinetic profile of the molecule remains invariant, yet the aerosolization technique influences deposition patterns within the lower airways. A soft‑mist system, by virtue of generating particles with an MMAD of approximately 1.5 µm, theoretically enhances peripheral lung penetration relative to dry‑powder formulations with larger particle sizes. Nonetheless, the empirical data derived from head‑to‑head trials have demonstrated only marginal differences in forced expiratory volume improvements, thereby questioning the clinical significance of such physicochemical distinctions. Moreover, the economic ramifications of prescribing a non‑PBS‑listed inhaler must be evaluated within the broader context of health‑system budgets and patient out‑of‑pocket expenditures. The environmental impact, whilst sometimes cited, should be quantified using lifecycle assessment methodologies rather than anecdotal assertions. Consequently, clinicians ought to prioritize device usability and patient preference, supported by robust evidence, to optimise adherence and therapeutic outcomes. In summary, the purported superiority of Tiova lacks a decisive advantage that would justify its higher price absent compelling patient‑specific factors.
Patrick Hendrick
October 12, 2025 AT 01:10The soft‑mist technology offers a smoother aerosol; it reduces inspiratory effort, which benefits many patients; the refillable option also cuts waste, and the clinical efficacy mirrors that of Spiriva.
abhishek agarwal
October 12, 2025 AT 01:20Absolutely, the reduced inspiratory demand is a game‑changer, yet it is equally vital to acknowledge that the single‑use cartridge model can impose a recurring logistical hurdle for patients living far from pharmacies, so the overall convenience calculus must weigh both aspects.
Michael J Ryan
October 12, 2025 AT 01:30Interesting points on device choice; just a quick note-when you write “patients’ lived experience” the apostrophe should follow the plural “patients”, not the singular, ensuring clarity in your nuanced discussion.
Khalil BB
October 12, 2025 AT 01:40In the theatre of inhalation, the mist is the silent oracle that bridges breath and healing.
Keri Shrable
October 12, 2025 AT 01:50The Tiova’s cloud dances like a soft sunrise over the bronchi, sprinkling tiny droplets that whisper relief; for those who cherish a gentle touch, it feels like a feathered kiss, while the classic Spiriva hums a steady drumbeat of familiarity, each inhalation a note in the symphony of daily management.
Destiny Hixon
October 12, 2025 AT 02:00Tiova is just another overpriced gimmick
mike brown
October 12, 2025 AT 02:10All this hype ignores the fact that most patients simply need a reliable dose, and whether it’s soft‑mist or dry powder matters far less than consistent adherence.
shawn micheal
October 12, 2025 AT 02:20It’s heartening to see the community dissecting each nuance, because at the end of the day the goal is to empower individuals to breathe easier; whether you gravitate toward Tiova’s sleek mist or Spiriva’s proven track record, the shared commitment to better health unites us all.
Stephen Jahl
October 12, 2025 AT 02:30From a pharmacoeconomic perspective, the incremental cost‑effectiveness ratio (ICER) of Tiova relative to Spiriva fails to surpass the willingness‑to‑pay threshold established by national health technology assessments, thereby rendering its adoption suboptimal in the absence of demonstrable superiority in patient‑reported outcome measures (PROMs). Moreover, the device’s aerosol dynamics-characterized by a low spray velocity and high fine‑particle fraction-necessitate rigorous real‑world adherence monitoring to validate the projected marginal gains in exacerbation reduction. Consequently, stakeholders must integrate health‑economic modelling, device ergonomics, and longitudinal safety data before endorsing a systematic shift in formulary placement.
gershwin mkhatshwa
October 12, 2025 AT 02:40Hey folks, just a reminder that whichever inhaler you end up with, the key is to stick with it, keep your technique sharp, and lean on your pharmacy or support group if you hit a snag-consistency beats perfection every time.